Sodium (Na) is a mineral that can build up to levels that have a negative impact on pasture soil quality, i.e. sodic soils (Figure 1 and Figure 2). These sodic soils can negatively affect pasture growth. When sodic soils occur, they need to be actively managed in order to reduce the Na levels.
Figure 1: An example of a sodic soil (Diagram by Kitty Terwolbeck / cc)
Figure 2: Another example of a sodic soil (Photo by Sandra Chile on Unsplash)
Excessive Na levels (>100 ppm and >5% contribution to base saturation) cause structural problems in the soil. This happens through soil dispersion, which is when wet clay particles move so far apart that they become totally separated. When this happens, soil aggregate stability is weakened, soil pores close up and the structure collapses. Water and nutrient storage is further limited, the soils ability to provide oxygen (O2) is reduced and a dense soil surface layer is formed1,2.
Excessive Na can be leached out of the system in a natural way, but the process takes a very long time. The process involves irrigating a lot more, which is not always possible, planting Na tolerant crops and high inputs of organic matter and/or manure. Leaching of excessive Na can also be improved by applying soil amendments in the form of soluble calcium though (Ca)1,2. Different forms of soluble Ca that can be applied to the soil include materials such as gypsum, Oasis and Calsap.
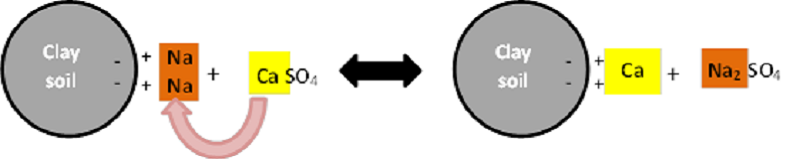
Gypsum is effective at washing out Na, because it contains calcium sulphate (CaSO4). The Ca ions in Gypsum displace Na ions on the exchange sites of soil clay particles. The displaced Na is free to bind with the sulphate (SO4) ions and form a leachable sulphate salt (Na2SO4) (Figure 3).
Figure 3: Na displacement by Ca on the exchange sites of soil clay particles
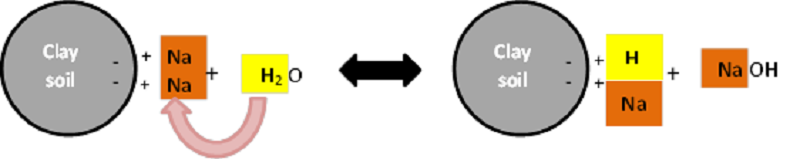
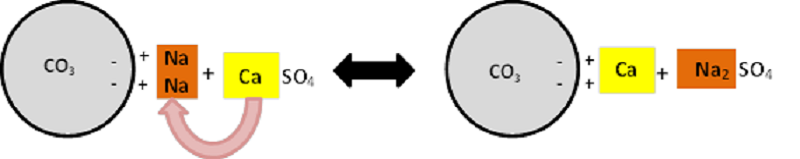
Water is also important in the leaching process in order for Na to be washed out of the system. Water contains H ions that are able to replace Na on the exchange sites of soil clay particles and bring Na into the soil solution (Figure 4). The Ca in Gypsum also has the ability to displace Na that is locked up in carbonate salts (e.g. Na2CO3) and form a leachable sulphate salt (Na2SO4) (Figure 5).
Figure 4: Na displacement by H on the exchange sites of soil clay particles
Figure 5: Na displacement by Ca on carbonate salts
Ca is able to displace Na on the exchange sites of soil clay particles and carbonate salts, because Ca has a stronger bond than Na. The order of strength of bond to the exchange sites for soil clay particles for different minerals is Mg>Ca>Na>K>H. Ca and Mg are thus not easily exchanged on the exchange sites for soil clay particles and form very stable bonds.
References:
1. Food and Agriculture Organisation, 2015. Sodic soils and their management (http://www.fao.org/docrep/x5871e/x5871e05.htm). Accessed 7 December 2015.
2. NSW Department of Primary Industries, 2015. Chapter D5. Sodic soil management. Vegetable SOILpak, 1-6.
- Milk, is it really just milk or so much more to it? - 2018-05-25
- Upside down thinking - 2018-04-19
- Happy soil life, happy grass, happy cows - 2018-04-04