Soils with excessive sodium and magnesium concentrations can negatively affect plant growth and productivity. Sodium and magnesium can accumulate in the soil to excessive levels due to: a) irrigation with water with high salt contents; b) excessive application of fertilizers containing these minerals; and C) deposition of animal excreta from grazing animals, which contain high levels of sodium and magnesium.
Excessive sodium levels: These soils have a high concentration of sodium ions (Na+), which can cause soil structure problems, reduce water infiltration, and increase soil alkalinity. High levels of sodium can also interfere with the uptake of essential minerals by plants, such as calcium and potassium. Soils with excessive sodium levels are often found in regions with high evaporation rates, low rainfall, and poor drainage, where salts (such as sodium) can accumulate in the soil.
Excessive magnesium levels: These soils have a high concentration of magnesium ions (Mg2+), which can negatively affect plant growth. High levels of magnesium can cause soil structure problems (compaction), reduce water infiltration, and increase soil alkalinity, similar to sodium. However, excessive magnesium can also lead to the imbalance of other minerals in the soil, such as calcium and potassium, which can negatively affect plant growth and productivity.
Soils with excessive levels of both sodium and magnesium can be managed through a combination of soil amendments which are discussed comprehensively below.
PRACTICAL MANAGEMENT STRATEGIES FOR EXCESSIVE SODIUM AND MAGNESIUM LEVELS
To remedy soils with high levels of magnesium and sodium, several approaches can be taken, such as aeration (i.e., ripping), creating proper drainage, the application of gypsum and sulphur and increasing soil organic matter. These practices can help remove sodium and magnesium from soils, each through a different mechanism. These practices can be implemented individually or concurrently depending on the context of the situation in the soil.
Aeration
Soil aeration refers to the process of introducing air into the soil and breaking up poor soil structure through ripping with a subsoil aerator. When soil is aerated, the oxygen in the air reacts with the minerals in the soil, causing chemical reactions that can break down the bonds between sodium and magnesium ions and the soil particles. This can make it easier for these ions to move out of the soil and into the surrounding environment, such as through leaching.
Drainage:
When soil drainage is improved, excess water drains from the soil, carrying with it sodium and magnesium ions.
As water moves through the soil, it can dissolve these ions, allowing them to be carried away from the root zone and into the surrounding environment. This process is called leaching. Therefore, by improving soil drainage, excess water can be removed, allowing for more efficient leaching of sodium and magnesium ions. As a side note, aeration also helps with this process.
It is important to note that excessive leaching can also lead to the loss of other essential minerals, such as nitrogen and potassium. Therefore, it is important to monitor soil fertility and nutrient levels and apply appropriate fertilizers and amendments as needed to maintain optimal soil health and plant productivity.
Gypsum
Gypsum is a naturally occurring mineral that can be added to soil to improve its structure and reduce the concentration of sodium ions. When gypsum is added to soil, it reacts with the sodium ions, forming a compound called sodium sulphate. This compound is soluble and can be easily leached out of the soil. As a result, the concentration of sodium ions in the soil is reduced.
Sulphur
Sulphur can also help to reduce the concentration of sodium and magnesium ions in soil. When sulphur is added to soil, it reacts with the water and oxygen in the soil to form sulfuric acid. This acid can break down the bonds between sodium and magnesium ions and the soil particles, making it easier for them to be leached out of the soil. Additionally, sulphur can also help improve the availability of other minerals in the soil, such as phosphorus, a welcome knock-on effect given that soils that are high in sodium and magnesium precipitate phosphate ions making it unavailable for the plants.
Soil carbon
Firstly, soil carbon can improve soil structure by binding soil particles together, creating pore spaces for air and water movement, and increasing the soil’s ability to hold onto minerals. This improved soil structure can help to reduce the adverse effects of excessive sodium and magnesium, such as soil compaction and reduced water infiltration. Secondly, soil carbon can also play a role in improving plant health and resilience. Carbon-rich soils tend to support a more diverse and abundant soil microbiome, which can help to promote plant growth and protect against soil-borne diseases. In addition, soil carbon can act as a buffer against fluctuations in soil pH, which can be particularly important in soils with high levels of sodium and magnesium, which can cause the soil to become more alkaline.
Overall, soil carbon can help to mitigate the negative impacts of excessive sodium and magnesium on soil and plant health, by improving soil structure, increasing nutrient availability, and promoting a healthy soil microbiome.
EXAMPLE OF A SALT-AFFECTED FARM IN THE COOKHOUSE
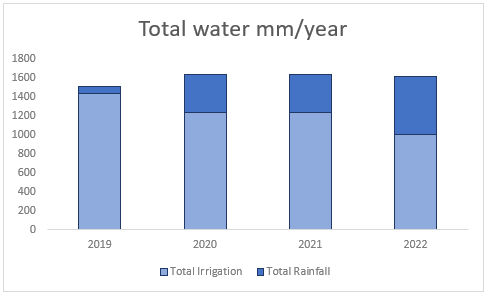
Farm A is located in the Cookhouse region in the Eastern Cape. The farm is in a semi-arid region with an average annual rainfall of less than 500mm per year. The region also has high evapotranspiration rates and inherently low soil organic material. The annual allocation of irrigation water ranges between 1000mm to 1400mm/ha/year and the entire operation is 100% irrigation. Farm A obtains its irrigation water from the Klein-vis River.
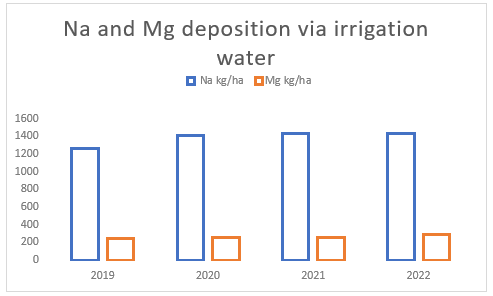
The water quality of the irrigation water used on the farm is very poor with excessive levels of sodium and magnesium. The average annual import of sodium from irrigation water is about 1 387 kg Na/ha while magnesium averages 263 kg Mg/ha(see Figure (b) ).
(a) Total water falling on these soils from irrigation and rainfall between 2019 and 2022 in mm/year
(b) Irrigation water quality between 2019 and 2022
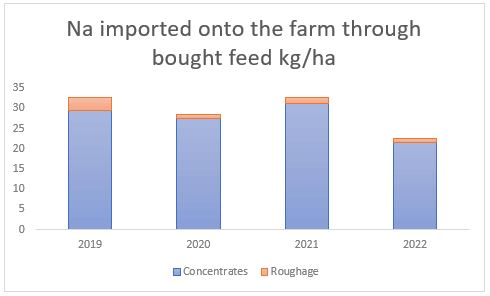
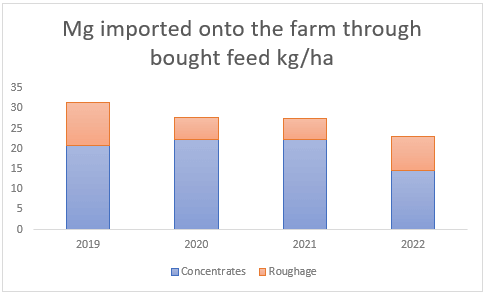
Furthermore, there is more deposition of these minerals via animal excreta. Since dairy farms feed concentrates in the dairy parlour, this introduces new nutrients into the farm system in the form of animal excrement. On average, sodium and magnesium brought in via concentrated feed annually result in 27 kg Na/ha and 20 kg Mg/ha (see Figures (c and d) ). Bought roughage, which is used to supplement pasture intake during times of pasture shortage, on the other hand, only brings in the smaller amount of 2 and 7 kg/ha(see Figures (c and d) ) of sodium and magnesium respectively.
The only active and productive removal of nutrients from the farm system is through the milk and meat that are sold from the farm. An average of only 5% of the sodium and 8% of the magnesium imported into the farm system is removed in this manner each year. Since there is such little removal through milk and meat, it is assumed that the rest is deposited back onto the pastures by the grazing animals in their excrement.
(c) Sodium introduced through bought feed between 2019 and 2022, much of which is excreted by the animals onto the dairy pastures.
(d) Magnesium introduced through bought feed between 2019 and 2022, much of which is excreted by the animals onto the dairy pastures.
Due to excessive nutrient loading, coupled with low removal rates of the salts, and poor precipitation rates, the soils have thus accumulated excessive amounts of sodium and magnesium. To remedy this, the farm has been on a long-term journey of using a combination of the management strategies mentioned above to mitigate the salt-affected soils.
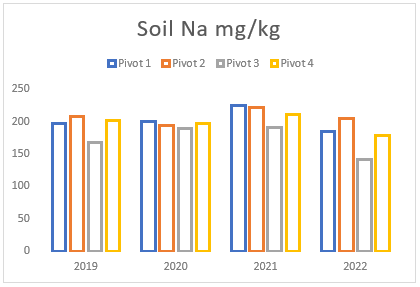
(e) Average Sodium levels of pivots 1 to 4 from soil samples taken between 2019 and 2022.
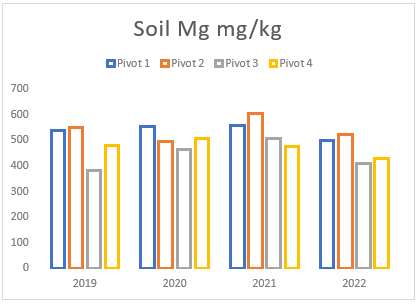
(f) Average Magnesium levels of pivots 1 to 4 from soil samples taken between 2019 and 2022.
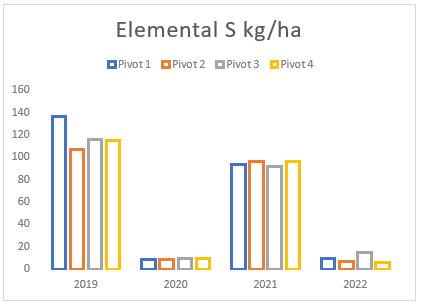
(g) Total sulphur applied from gypsum and elemental sulphur on pivots 1 to 4 between 2019 and 2022.
Throughout the years, Farm A has experienced a consistent but gradual increase in sodium and magnesium levels across all pivots (see Figures (e and f)). To address this issue, two applications of elemental sulphur and gypsum were implemented on these pivots in 2019 and 2021 (see Figure (g)). However, the aeration process on the pivots was not conducted adequately until the autumn and spring of 2021. In previous years, there was a time gap between ripping the soil and applying gypsum/sulphur. This resulted in either applying gypsum to non-aerated soils or conducting ripping without immediate application of gypsum and/or sulphur. In contrast, during the autumn and spring of 2021, these strategies were carried out simultaneously, deviating from the approach of the previous years.
Despite the substantial addition of sodium and magnesium through irrigation and feed, the soil’s salt levels remained relatively steady (see Figures (e and f)). However, in 2022, a notable reduction in both minerals was observed due to the implementation of the process mentioned above, that is aeration, gypsum and sulphur application, and prompt drainage. These management strategies facilitated the acceleration of the drainage and leaching processes, effectively mitigating the excessive sodium and magnesium in the soil. These findings emphasize the significance of using a combination of the abovementioned methodologies, as discussed earlier in the article, for effectively addressing elevated sodium and magnesium levels in the soil.
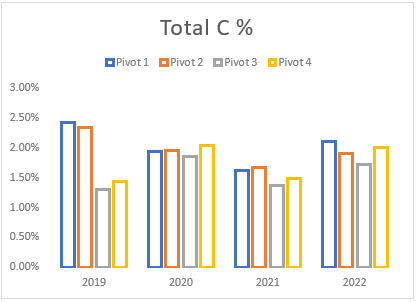
(h) Average Total carbon levels of pivots 1 to 4 from soil samples taken between 2019 and 2022.
Although the subsoil ripping (aeration) in 2021 resulted in the decline of soil carbon, the decline was generally small and short-lived as increases were observed in the 2022 soil analysis. This process can provide several benefits, such as improving soil structure, reducing soil compaction, and increasing nutrient availability. However, aerating soil can also temporarily reduce soil carbon. Soil carbon is primarily stored in the form of organic matter, which is made up of dead plant and animal material that has not yet decomposed. When soil is aerated, the oxygen in the air stimulates microbial activity, which results in an increase in the rate of decomposition of organic matter.
This increased decomposition rate can lead to a temporary reduction in soil carbon levels. The increased microbial activity stimulated by aeration can also lead to an increase in the availability of minerals such as nitrogen and phosphorus, which can be beneficial for plant growth. In addition, aeration helps to stimulate the growth of plant roots, which leads to an increase in the amount of organic matter produced by plants over time. Therefore, while aeration temporarily reduces soil carbon levels, it ultimately leads to an increase in organic matter production and storage in the soil, as well as improved soil health and plant growth.
Conclusion
Soils with excessive levels of both sodium and magnesium can be managed through a combination of soil amendments, such as the application of gypsum and sulphur, building soil organic matter levels, and drainage and aeration. It is important to note that annual soil testing is recommended to determine the best course of action for your specific situation and to monitor improvements (or lack thereof) from strategies applied.
- The management of soils with excessive sodium and magnesium levels - 2023-06-12
- Understanding evapotranspiration better - 2021-10-18
- Soil fungi connections - 2021-09-28