Soil respiration has been extensively promoted as a simple, holistic measure of microbial activity in the soil. Simply capture and measure the amount of carbon dioxide produced by soil and you will have an idea of the metabolic activity of the life in the soil. Sounds simple, which is exactly why we have been measuring soil respiration over the past three years. After much trial and error, reading and consulting with various other people also measuring soil respiration, we settled on a soil respiration methodology based on Parkin et al. (1996). Basically 10ml of 1M sodium hydroxide is used to capture carbon dioxide released from a 20g air-dried soil sample that is later rewetted (to field water holding capacity judged by the eye) and stored in an air-tight 1l (as shown in the picture above) container over three days in an incubator at 25 degrees Celsius. After 72 hours the sodium hydroxide sample is removed from the jar and 2ml of 1M barium chloride is added to it in order to precipitate the carbonate. Three drops of phenolphthalein is further added to this sample as an indicator, giving it a pink colour. Hydrochloric acid (0.5M) is then used to titrate the sodium hydroxide solution, with the volume of hydrochloric acid needed to change the sample colour from pink to clear being recorded. The amount of hydrochloric acid used to change a control sample (also 10ml of 1M sodium hydroxide, but where no soil was added) is subtracted from the amount of hydrochloric acid used by each sample in order to determine the total amount of carbon dioxide captured resulting from the soil over the three days.
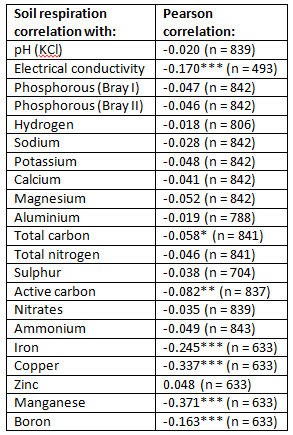
The results raised a question we had to ask ourselves, and which we now ask to any other interested party. How relevant is soil respiration as a measure of soil life? We analysed well over 800 samples from pasture soils in the Eastern Cape, across varying management systems, climate regions, soil types and pasture crop combinations. The table below shows the results of the soil respiration analysis correlated with various other measures of soil health. The only measures which showed any correlation to soil respiration were electrical conductivity, total carbon, active carbon, iron, copper, manganese and boron. They all had a negative correlation, meaning that as the soil respiration values increased, these measures decreased in value, and vice versa. This does not make any sense according to our understanding of soil life, especially with regards to total and active carbon. These are both reliable indicators of soil life, and for them to be negatively correlated with soil respiration does not inspire any confidence in soil respiration as an indicator of soil life.
Based on these findings, we have decided to discontinue analysing our soil samples for soil respiration. This is until someone can point out to us what we might have missed in this supposedly great measure of soil life that is soil respiration.
As a result of this process, the main questions we have are:
- Is the method of measuring soil respiration by titration an accurate measure of soil respiration?
- Why is there no correlation between the soil respiration results and other indicators of soil life (e.g. total carbon and active carbon)?
- How does the measurement of soil respiration contribute to a greater understanding of the current soil life/biology situation?
- What contribution does the measurement of soil respiration have to soil management and improving soil health?
References:
- Parkin TB, Doran JW and Franco-Vizcaino E. 1996. Field and laboratory tests of soil respiration. In: Doran JW and Jones AJ (eds). Methods for assessing soil quality. SSSA Special Publication Number 49. Soil Science Society of America, Inc., Madison, Wisconsin, USA, pp 231-245.
- A carbon footprint assessment for pasture-based dairy farming systems in South Africa - 2024-02-07
- What progress have farms participating with Trace & Save made over the past 10 years? - 2023-09-06
- Carbon footprint reduction over time: Lessons from pasture-based dairy farms in South Africa - 2023-09-04